We’ve all heard the buzzwords: “mitochondrial dysfunction,” “nervous system dysregulation,” “histamine intolerance.” It can feel like just another thing to fix. But really, it comes back to this:
A healthy body is made of systems → built from organs → made of tissues → made of cells.
Inside each cell, the mitochondria decide: Do we grow, repair, conserve, or defend?
They don’t just make ATP — they sense danger, direct immune responses, buffer calcium, regulate hormones, and even control apoptosis.
That’s why if you’re struggling with chronic conditions, fatigue, brain fog, or reactivity, the mitochondria are always part of the story.
Below is a practical, science-forward guide, so you can look at routine labs and spot patterns that suggest your mitochondria want help. We’ll weave in bioenergetics, psychoneuroimmunology, and quantum biology (light, water, magnetism), then finish with a lab-by-lab checklist.
Quick note: This is education, not diagnosis. Use it to ask sharper questions and tailor next steps with your clinician.
Mitochondria 101: The Jobs
ATP production (OxPhos):
The classic role of mitochondria is oxidative phosphorylation (OxPhos), where electrons from food (glucose, fats, ketones) are combined with oxygen to make ATP — the “energy currency” of life. But it’s not just energy: every ATP molecule is bound to magnesium (Mg-ATP), and ATP also serves as a signaling molecule for things like pain, immune activity, and cell-to-cell communication. When ATP levels drop, cells go into conservation mode, which you feel as fatigue.Redox & ROS signaling:
The electron transport chain produces reactive oxygen species (ROS) like superoxide and hydrogen peroxide. These aren’t just “toxic byproducts” — in low to moderate amounts, they are signaling molecules that tell your body to adapt (exercise benefits, immune priming, new mitochondria creation). When ROS overwhelm antioxidant defenses, they shift from helpful messengers to damaging agents — oxidizing proteins, lipids, and DNA. The balance between oxidation (ROS) and reduction (antioxidants) is what’s called your redox state, and it’s a central marker of health.Apoptosis & danger sensing (Cell Danger Response):
Mitochondria are the “safety officers” of the cell. When they sense too much stress (toxins, infection, trauma), they release signals like cytochrome c to initiate apoptosis (programmed cell death). This isn’t random destruction — it’s a protective measure to prevent damaged cells from becoming cancerous or spreading infection. They can also broadcast the Cell Danger Response (CDR): shutting down growth, stiffening membranes, and sending “not safe” signals to the immune system. Mast cells, for example, are tuned to these signals and may degranulate (release histamine and other mediators) when mitochondria say “the terrain isn’t safe.”Calcium buffering:
Calcium is a universal signal for nerves firing, muscles contracting, and hormones releasing. Mitochondria act as sponges for calcium, absorbing excess so cells don’t overload. If calcium buffering fails, you can get symptoms like muscle spasms, arrhythmias, migraines, or anxiety — because calcium signaling has gone chaotic. This buffering also regulates the opening of mitochondrial pores that decide between survival and apoptosis.Steroidogenesis:
The very first step of hormone synthesis — converting cholesterol into pregnenolone — happens in the inner mitochondrial membrane. Without healthy mitochondria, your body can’t make cortisol, estrogen, testosterone, progesterone, or DHEA efficiently. This is why mitochondrial stress often shows up as hormone imbalance or adrenal fatigue-like symptoms.Thermogenesis:
In brown fat cells, mitochondria express proteins called uncoupling proteins (UCPs). Instead of making ATP, they let protons “leak” across the membrane, generating heat. This helps regulate body temperature, metabolic rate, and resilience to cold exposure. It’s also why practices like cold plunging can stimulate mitochondrial biogenesis and fat metabolism.Innate immunity crosstalk:
Mitochondria are ancient bacteria that merged with our cells. Because of that origin, their DNA (mtDNA) looks bacterial to the immune system. When mitochondria are damaged, fragments of mtDNA leak into circulation and the immune system reads them as danger signals, ramping up inflammation. This links mitochondrial health to autoimmune disease, chronic inflammation, and trauma biology.Membrane architecture (Cardiolipin):
Cardiolipin is a unique phospholipid found almost exclusively in mitochondrial membranes. It literally anchors the electron transport chain complexes in place. If cardiolipin is oxidized (by excess ROS, toxins, or nutrient deficiencies), the ETC falls apart, ATP drops, and the mitochondria may even initiate apoptosis. Nutrients like phosphatidylcholine and antioxidants like CoQ10 or melatonin help protect cardiolipin, maintaining both energy production and cell survival.
Metabolism Map
1) Glycolysis → Pyruvate
Glucose splits to make pyruvate (a little ATP, a little NADH). If oxygen delivery or mitochondrial entry is limited, pyruvate turns into lactate (that “burn” feeling).
Key nutrients: B1, B3, Mg, P.
2) Pyruvate Dehydrogenase (PDH) → Acetyl-CoA
PDH is the gatekeeper into mitochondria. It needs B1, B2, B3, B5, lipoic acid, Mg. Infections, inflammation, or stress hormones often inhibit PDH, forcing a lactate build-up (Warburg-like shift).
3) Krebs / Citric Acid Cycle
Acetyl-CoA spins the cycle to generate NADH/FADH₂ for the ETC.
Nutrient highlights: B1, B2, B3, B6, Mg, Mn, lipoic acid, CoA (B5); iron-sulfur clusters; biotin (carboxylases).
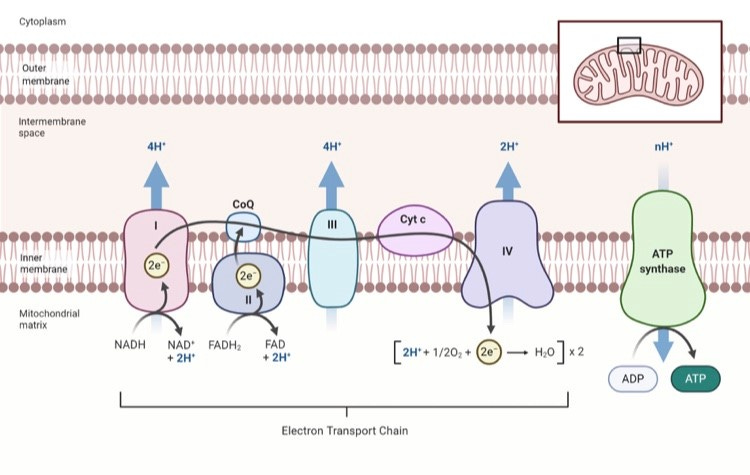
4) Electron Transport Chain (ETC)
Complex I (iron-sulfur; NADH)
Complex II (succinate dehydrogenase; FAD/B2)
CoQ10 shuttles electrons to
Complex III (cyt b/c1; iron-sulfur) → cytochrome c →
Complex IV (cytochrome c oxidase; copper-dependent) uses oxygen as the final electron acceptor → pumps protons →
ATP Synthase makes ATP.
When the ETC is running, it also builds Exclusion Zone (EZ) water in and around the mitochondrial matrix (supporting charge/voltage and protein folding), and produces carbon dioxide (CO₂) as a byproduct of respiration—CO₂ then supports oxygen delivery (Bohr effect) and pH buffering.
The ETC is photoresponsive: cytochrome c oxidase (Complex IV) is stimulated by red and near-infrared light roughly 600–980 nm, which can increase electron flow, oxygen consumption, and ATP output.
Needs: Oxygen, iron, copper, sulfur clusters, CoQ10, Mg, cardiolipin, phosphatidylcholine, adequate thyroid hormone and redox balance.
Oxygen, CO₂, and Why Breathing Patterns Matter
Hemoglobin drops off oxygen best when CO₂ is adequate (Bohr effect). Chronically low CO₂ (from overbreathing/anxiety or metabolic depletion) can make hemoglobin hold oxygen too tightly → functional hypoxia (tissues starve despite “normal” SpO₂).
On a CMP, “CO₂” = bicarbonate (HCO₃⁻) (a metabolic buffer), not lung CO₂. Low bicarb (~<24 mmol/L) can reflect acid load (lactate, ketones), mineral depletion, diarrhea, renal losses—or chronic respiratory alkalosis compensation. Patterns matter more than a single value.